Well before Darwin, naturalists noted the tendency of living organisms to continually generate diversity at all scales, generation after generation. Mostly through mutation, variation appears in individuals and is then spread through conjugation (in prokaryotes) or sexual reproduction (in eukaryotes). Indeed, diversity is one of the enduring themes of life, providing populations with potential ecological resilience and insulation from unpredictable futures, as the combination of alleles, morphologies, and behaviors that result in relative reproductive success are propagated, a concept called biological fitness. Sexual reproduction evolved as a means to spread and recombine diversity in eukaryotes. Accordingly, it should come as little surprise that biology related to sex itself exhibits substantive variety, including diverse sexual morphologies, a range of sex-influenced behaviors, and varied reproductive strategies.
The major clades of eukaryotes – plants, animals, fungi, and the many kingdoms of protists – have evolved both unique and shared aspects in their sexual reproductive mechanisms, but one such aspect – the differentiation of gametes into two major forms – is a common theme. Anisogamy, the property of having two types of gametes - one very large and relatively immotile and one very small and highly mobile – is a key feature of sexual reproduction in all animals, all land plants, and many protist kingdoms.
For almost two centuries, many biologists have used gametic sex (sex as it relates to gametes only) as a proxy for biological sex (sex as it relates to bodies, behaviors, populations, and species). This use of gametes as the sole determinant of sex results in a perspective in which all animals seem to fit neatly into two categories, male or female, based only on which gamete they make. Because gametes are binary in animals, reliance on gametic sex often results in assumptions that the morphologies, behaviors, and strategies related to sex in animals are also best characterized as binary. In our view, this binary classification of sex in animals is insufficient for capturing the full breadth of biological sexual diversity.
Some of the inadequacies of the binary sex classification for individuals are uncontroversial, as it has long been known that a large number of species – around 20% of non-arthropod invertebrates – include individuals that are simultaneously hermaphroditic. Many others, including around 2% of vertebrates, are sequential hermaphrodites. Animal bodies exist in a variety of sexed forms, with some even reconfiguring their biology relating to sex, including for the production of gametes, within their individual life history, sometimes multiple times. The presence of simultaneous and sequential hermaphrodites vexes the binary classification for sexed bodies and demonstrates that sex is neither immutable nor neatly reducible to gamete production.
Furthermore, sexual dimorphisms, sexual bimodalities, and a spectrum of sex-influenced gene expression are observed throughout animal bodies and across animal species. Some of this variation is patterned in close association with gamete production, but much is not so simply described. Across bodies, behaviors, and physiologies, there is substantive inherent variety and diversity, creating a sexual continuum of genetic, developmental, and behavioral biology within and across species. Individual animals can vary widely in the development, patterning, and expression of sexual biology in a variety of ways, from body sizes and compositions, to color patterns and genital anatomy, to courtship behaviors and parental investment, to name some of the most commonly diverse components of sex. These biological variations rarely collapse into two discrete sex-based categories defined by gamete production. Moreover, much of the biological variations in bodies, even those closely associated with reproduction, are also engaged in a diversity of other bodily functions and processes with myriad phylogenetic, ecological, and behavioral constraints and affordances, which are also not ubiquitously or consistently associated with the type of gametes a body produces.
Dramatic sexual diversity and variation is not limited to adulthood. There is also substantive diversity in mechanisms of sex development across various animal taxa. There are chromosomal systems, other genetic systems, as well as systems based on season, temperature, age, social status, and population density, most of which have convergently evolved in multiple disparate lineages, emphasizing the relative genetic, cellular, and developmental flexibility and adaptability of these sex systems. Importantly, the emergence of diversity and variety throughout all levels of sex biology, from molecules to behaviors, has continued relentlessly since the first appearance of animals, even as the binary differentiation of gametes held steady. It must also be noted that sexual diversity – like all forms of variation – would tend not to persist in a population if it incurred substantial fitness costs.
As a result, across most animals, there is plenty of variation within sex categories and, quite often, this variation overlaps between sex categories, generating a continuum of sexual morphologies and behaviors that are often, but not always, bimodal, and rarely binary. Further still, variation among the various sexual morphologies isn’t always correlated. A particular animal may fall outside of its sex-typical range for one feature, but not for others. Each animal is a mosaic of sexual morphology, some dimorphic, some bimodal, some not, and the degree of correlation among the aspects of these morphologies can vary widely in different species. For this reason, we argue that gametic sex is but one aspect of biological sex, which also includes such distinctions as genetic sex, chromosomal sex, gonadal sex, genital-anatomical sex, morphological sex, hormonal sex, and so on.
Importantly, the recognition that sex can be a complex mixture of anatomy, physiology, and behavior does not serve to deny or minimize the existence and impacts of sex differences. In fact, it affirms them and emphasizes their importance. While the matter of which gamete an animal body makes – its gametic sex – is clearly important, it is not the only variable by which animal morphologies or behaviors can be, or are, sexed. If these other variables were neatly binary, immutable, and non-overlapping, it would not be necessary to distinguish between gametic sex and biological sex. But, since nearly all other sex traits are either continuous or bimodal, are not always immutable nor perfectly correlated, a simple and categorical definition of sex that is based purely on gamete production is both unwarranted and potentially misleading.
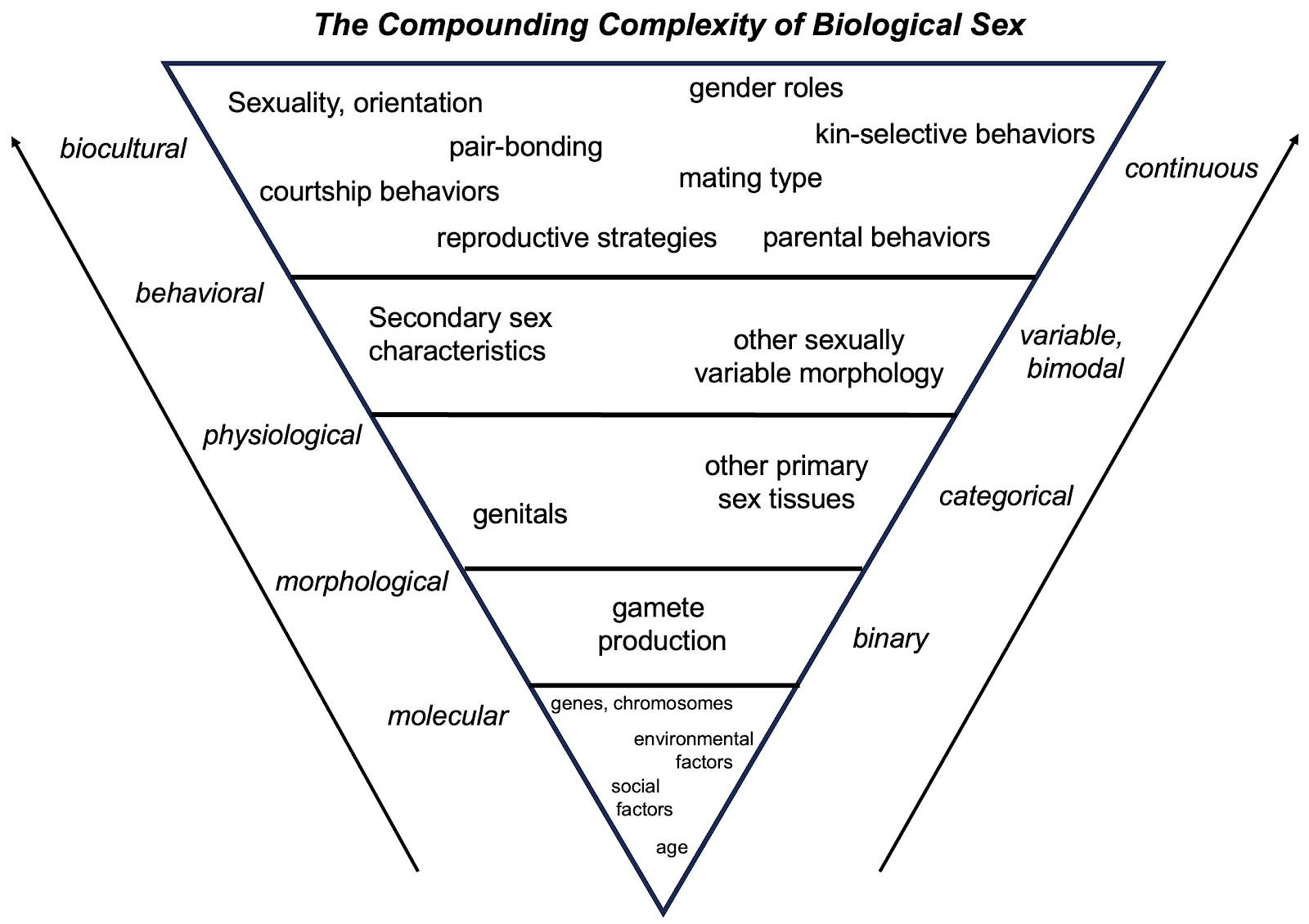
The non-categorical nature of sex is best illustrated by example, and our own species provides a wealth of them. As with other animals, humans have sexed bodies. However, most human biological variation is not discretely associated with which gamete one produces. Rather, most human variation is independent of sex. Further, sex-related variation is usually continuous, often bimodal, at times dimorphic, and almost never binary. In short, when assessing the distribution of human biological variation in the contexts of health, disease, daily life, and social life, one’s gametic sex is often inconsequential relative to the majority of other variation, processes, and patterns, both sex-related and not.
Animal morphology and physiology are the product of complex interactions of biological, developmental, and environmental systems, and the human environment is a particularly complex assemblage of biotic and abiotic factors: what we refer to as human culture. Human phenotypic expression is always mutually shaped by cultural milieu. It is well-established that adult height and weight, childhood development trajectories, taste bud reactivity, muscle development and coordination, patterns of sexual arousal, resistance (or lack thereof) to disease-causing bacteria, and nearly every other aspect of human bodies emerge from mutual and interactive development of physiology, morphology, cultural context, and lived experiences. Basic perceptual processes such as smell, color vision, sound, and taste are mutually shaped by physiology and cultural experience. The traits often associated with sex in humans: femininity, masculinity, sexuality, sports abilities, child-care, health, body shape, how one speaks, how one walks, etc., are products of dynamic relationships that interweave the biological and the cultural, as well as the historical, into the individual organism. While humans are, of course, biological organisms, the totality of the human experience, including our bodies, cannot be reduced to either specific innate (biological) or external (environmental/cultural) influences; it is a synthesis of both; humans are biocultural.
It goes without saying that an ovum is not a woman and a sperm is not a man. However, the assumption that patterns of variation and difference in humans are primarily explained by this one solitary aspect of sex biology remains common. If biological sex is to have comprehensive meaning for the entirety of the human experience, defining it based solely on gamete production typology is oversimplified and scientifically incorrect. All of the biological variation associated with sex is important and plays a major role in how any given individual engages with their body and the cultural dynamics of their society. Such relations are neither simple nor uniform across time and geography, nor binary. Human sex is biocultural.
Besides genital anatomy, some of the more well-known sex-related differences in human bodies include aspects of height, weight, pelvic girdle anatomy, fat distribution, and upper-body strength. More subtle variations include metabolic rates, hormone ratios, and bone density, among other elements. Importantly, in most of these measures, the variation within gamete-defined sex groups is larger than the difference between them, and substantial overlap is present across all studied populations. Thus, these variable features, even when dimorphic, match the textbook definition of continuous variation, with some exhibiting a markedly bimodal distribution and others hardly at all. And, a given individual may fall outside of the sex-typical range in one or more measures, but not in others. Humans are a mosaic of sex related traits; the degree of concordance among them varies widely, and the developmental dynamics related to all are complex.
Furthermore, it is not currently known which, or how much, of all of this patterned variation is shaped by differences in how boys and girls, and men and women, use their bodies on a daily basis. While human anatomical development is a fairly canalized pathway producing a relatively consistent phenotypic range, the developmental process itself both affects and is substantively affected by how that anatomy is physically and socially engaged, especially during childhood and adolescence. Indeed, there is emerging evidence that persistent culturally mandated gender differences in play behaviors and sports participation, which are quite substantial in many cultures, have clear and strong effects on the developmental dynamics of skeletal and muscle formation.
Similarly, gendered differences in the social environment likely contribute to differences in sexed bodies in ways that are probably impossible to untangle. For example, it is well established that hormone levels and ratios are affected by the social environment, and these same hormones directly impact both the development of many tissues and sex-related and non-sex-related behaviors (muscle hypertrophy, hair distribution, metabolism, mental alertness, and libido, to name a few). Such complexities are not limited to humans by any stretch, as Patricia Brennan explains in another essay in this series, in Ruddy Ducks, social interactions directly impact the seasonal growth and development of the penis, emphasizing the dynamically responsive nature of sexual anatomy, even in adult animals.
Importantly, sex-related variations in human bodies and the cultural dynamics in which these bodies exist may translate to gender-related differences in health and wellness in ways that we are only beginning to understand. Although men are diagnosed with heart attacks more frequently, women are more likely to die in the year after they experience one. Men are more likely to develop Parkinson’s Disease, while women are more likely to suffer from Lupus, among many other gender-related variations in autoimmune disease. Gender differences also emerge in rates of bipolar disorder, depression, and suicide. In all of these cases, there are biological, cultural, individual, and environmental variables involved, and their individual and synergistic roles in the development of these health challenges are far from simple or binary. Gender differences in health and disease do exist, and sex is a central feature in many, if not all, of them. However, other than infertility, it’s difficult to imagine a social or healthcare context in which gamete type or production matters much at all. At the end of the day, the biocultural realities of sex have far more impact on daily life, both the mundane and the profound, than which gamete one makes.
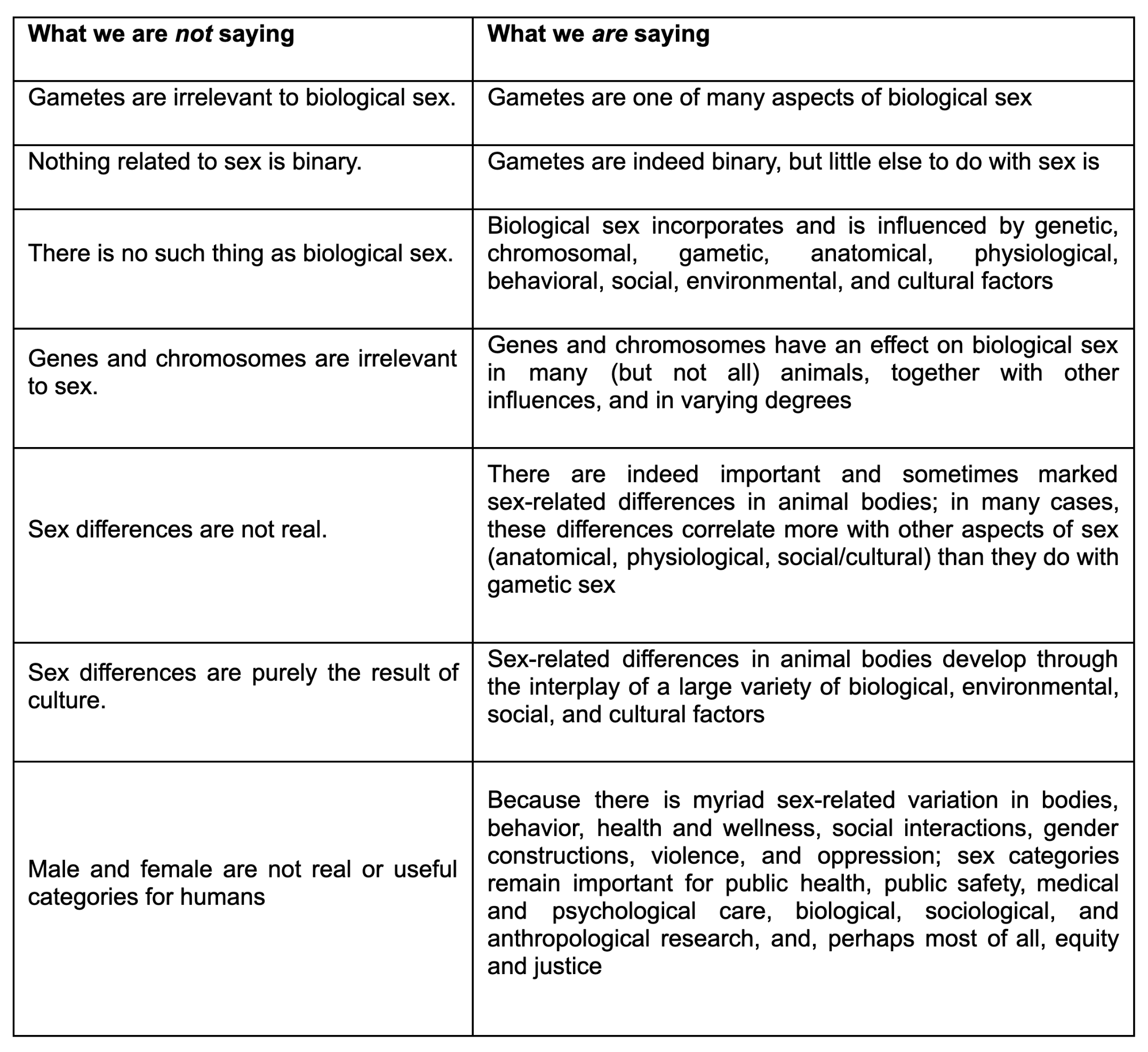
It has not escaped our notice that some of the alternative approaches to defining sex, including those that otherwise affirm the importance and ubiquity of sexual variation, continue to insist on the primacy of gametes in that definition. This may ultimately prove to be a purely semantic difference, rather than a conceptual one. Even so, because semantics can powerfully shape ideas and biases, there are both scientific and social reasons to favor a more comprehensive understanding of sex, despite the allure of a simple binary classification. In our view, reducing all sex-related biology to gametes not only ignores features throughout sexed bodies (which don’t always correlate with gametic sex), it also leaves no space for intersex individuals and emphasizes the narrative that there are only two kinds of bodies, which has impeded our scientific understanding of the prevalence and importance of sexual diversity.
At the end of the day, two things about sex are clear:
- Substantive research across multiple disciplines and foci emphasizes that the study of human biology must include diverse and often globally dynamic cultural processes. Differences in the experiences of being human, including gender and so many other elements, affect how sex is expressed and experienced in the body.
- Sex as an aspect of biological systems affects a multitude of human bodily processes, systems, and phenotypes.
Therefore, any explicit study of sex and gender and how they may or may not correlate with various “sex differences” in health outcomes, bodies, and lives must be undertaken in the context of both the full biological diversity of sex and the biocultural reality of humans. The best hope we have of improving our scientific understanding of the tangled realities of sex in humans is to look within and across cultures, bodies, and life histories and remember that we, too, are part of the rich breadth of biological sexual diversity on this planet.
Recommended Reading:
- Bohannon, C. (2023). Eve: How the Female Body Drove 200 Million Years of Human Evolution. Random House Canada.
- Cooke, L. (2022). Bitch: A Revolutionary Guide to Sex, Evolution and the Female Animal. Random House.
- De Waal, F. (2022). Different: Gender through the Eyes of a Primatologist. WW Norton & Company.
- Fausto-Sterling, A. (2000). Sexing the Body: Gender Politics and the Construction of Sexuality. Basic books.
- Fuentes, A. (2025). Sex Is a Spectrum: The Biological Limits of the Binary. Princeton University Press.
- Kaishian, P. O. (2025). Forest Euphoria: The Abounding Queerness of Nature. Spiegel & Grau.
- Lents, N. H. (2025). The Sexual Evolution: How 500 Million Years of Sex, Gender, and Mating Shape Modern Relationships. HarperCollins.
- Prum, R. O. (2023). Genes, Development, and Sexual Difference. In: Performance All the Way Down. University of Chicago Press.
- Richardson, S. S. (2019). Sex Itself: The Search for Male and Female in the Human Genome. University of Chicago Press.
- Roughgarden, J. (2013). Evolution's Rainbow: Diversity, Gender, and Sexuality in Nature and People. Univ of California Press.
Bibliography:
- Ah-King, M. (2013). On anisogamy and the evolution of ‘sex roles’. Trends in Ecology & Evolution, 28(1), 1-2.
- Arnold, C. (2016). The sparrow with four sexes. Nature, 539(7630).
- Bachtrog, D., Mank, J. E., Peichel, C. L., Kirkpatrick, M., Otto, S. P., Ashman, T. L., ... & Tree of Sex Consortium. (2014). Sex determination: why so many ways of doing it?. PLoS biology, 12(7), e1001899.
- Baskin, L., Shen, J., Sinclair, A., Cao, M., Liu, X., Liu, G., ... & Cunha, G. R. (2018). Development of the human penis and clitoris. Differentiation, 103, 74-85.
- Borsa, A., Miyagi, M., Ichikawa, K., Jesus, K. D., Jillson, K., Boulicault, M., & Richardson, S. S. (2024). The new genetics of sexuality. GLQ, 30(1), 119-140.
- Boulet, N., Briot, A., Galitzky, J., & Bouloumié, A. (2022). The sexual dimorphism of human adipose depots. Biomedicines, 10(10), 2615.
- Brown, G. R., Laland, K. N., & Mulder, M. B. (2009). Bateman's principles and human sex roles. Trends in ecology & evolution, 24(6), 297-304.
- Conley, A., Place, N. J., Legacki, E. L., Hammond, G. L., Cunha, G. R., Drea, C. M., ... & Glickman, S. E. (2020). Spotted hyaenas and the sexual spectrum: reproductive endocrinology and development. Journal of Endocrinology, 247(1), R27-R44.
- deMayo, B. E., Jordan, A. E., & Olson, K. R. (2022). Gender development in gender diverse children. Annual review of developmental psychology, 4, 207-229.
- DeCasien, A. R., Guma, E., Liu, S., & Raznahan, A. (2022). Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biology of Sex Differences, 13(1), 43.
- Downey, G., & Lende, D. H. (2012). Evolution and the brain. The encultured brain: An introduction to neuroanthropology, 103-138.
- DuBois, L. Z., & Shattuck‐Heidorn, H. (2021). Challenging the binary: Gender/sex and the bio‐logics of normalcy. American Journal of Human Biology, 33(5), e23623.
- Dunsworth, H. M. (2020). Expanding the evolutionary explanations for sex differences in the human skeleton. Evolutionary Anthropology: Issues, News, and Reviews, 29(3), 108-116.
- Eliot, L., Ahmed, A., Khan, H., & Patel, J. (2021). Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neuroscience & Biobehavioral Reviews, 125, 667-697.
- Fausto-Sterling, A. (2019). Gender/sex, sexual orientation, and identity are in the body: How did they get there?. The Journal of Sex Research, 56(4-5), 529-555.
- Garofalo, E. M., & Garvin, H. M. (2020). The confusion between biological sex and gender and potential implications of misinterpretations. In Sex estimation of the human skeleton (pp. 35-52). Academic Press.
- Glickman, S. E., Short, R. V., & Renfree, M. B. (2005). Sexual differentiation in three unconventional mammals: spotted hyenas, elephants and tammar wallabies. Hormones and Behavior, 48(4), 403-417.
- Hyde, J. S., Bigler, R. S., Joel, D., Tate, C. C., & van Anders, S. M. (2019). The future of sex and gender in psychology: Five challenges to the gender binary. American Psychologist, 74(2), 171.
- Joel, D. (2012). Genetic-gonadal-genitals sex (3G-sex) and the misconception of brain and gender, or, why 3G-males and 3G-females have intersex brain and intersex gender. Biology of sex differences, 3(1), 27.
- Joel, D., Berman, Z., Tavor, I., Wexler, N., Gaber, O., Stein, Y., ... & Assaf, Y. (2015). Sex beyond the genitalia: The human brain mosaic. Proceedings of the National Academy of Sciences, 112(50), 15468-15473.
- Greaves, L., & Ritz, S. A. (2022). Sex, gender and health: mapping the landscape of research and policy. International Journal of Environmental Research and Public Health, 19(5), 2563.
- Tombak, K. J., Hex, S. B., & Rubenstein, D. I. (2024). New estimates indicate that males are not larger than females in most mammal species. Nature Communications, 15(1), 1872.
- Krieger, N. (2020). Measures of racism, sexism, heterosexism, and gender binarism for health equity research: from structural injustice to embodied harm—an ecosocial analysis. Annual review of public health, 41(1), 37-62.
- McLaughlin, J. F., Brock, K. M., Gates, I., Pethkar, A., Piattoni, M., Rossi, A., & Lipshutz, S. E. (2023). Multivariate models of animal sex: breaking binaries leads to a better understanding of ecology and evolution. Integrative and Comparative Biology, 63(4), 891-906.
- DuBois, L. Z., Kaiser Trujillo, A., & McCarthy, M. M. (2025). Sex and Gender: Toward Transforming Scientific Practice (p. 327). Springer Nature.
- Leatherman, T., & Goodman, A. (2020). Building on the biocultural syntheses: 20 years and still expanding. American Journal of Human Biology, 32(4), e23360.
- Ocobock, C., & Lacy, S. (2024). Woman the hunter: The physiological evidence. American Anthropologist, 126(1), 7-18.
- Owens, I. P., & Hartley, I. R. (1998). Sexual dimorphism in birds: why are there so many different forms of dimorphism?. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1394), 397-407.
- Pape, M., Miyagi, M., Ritz, S. A., Boulicault, M., Richardson, S. S., & Maney, D. L. (2024). Sex contextualism in laboratory research: enhancing rigor and precision in the study of sex-related variables. Cell, 187(6), 1316-1326.
- Rauch, J. M., & Eliot, L. (2022). Breaking the binary: Gender versus sex analysis in human brain imaging. NeuroImage, 264, 119732.
- Roselli, C. E. (2018). Neurobiology of gender identity and sexual orientation. Journal of neuroendocrinology, 30(7), e12562.
- Smiley, K. O., Munley, K. M., Aghi, K., Lipshutz, S. E., Patton, T. M., Pradhan, D. S., & Solomon-Lane, T. K. (2024). Sex diversity in the 21st century: concepts, frameworks, and approaches for the future of neuroendocrinology. Hormones and behavior, 157, 105445.
- Todd, E. V., Liu, H., Muncaster, S., & Gemmell, N. J. (2016). Bending genders: the biology of natural sex change in fish. Sexual Development, 10(5-6), 223-241.
- Van Anders, S. M. (2013). Beyond masculinity: Testosterone, gender/sex, and human social behavior in a comparative context. Frontiers in neuroendocrinology, 34(3), 198-210.
- Van Anders, S. M. (2024). Gender/sex/ual diversity and biobehavioral research. Psychology of Sexual Orientation and Gender Diversity, 11(3), 471.
- Van Anders, S. M., Steiger, J., & Goldey, K. L. (2015). Effects of gendered behavior on testosterone in women and men. Proceedings of the National Academy of Sciences, 112(45), 13805-13810.
- Williams, J. S., Fattori, M. R., Honeyborne, I. R., & Ritz, S. A. (2023). Considering hormones as sex-and gender-related factors in biomedical research: challenging false dichotomies and embracing complexity. Hormones and behavior, 156, 105442.
- Zajitschek, S. R., Zajitschek, F., Bonduriansky, R., Brooks, R. C., Cornwell, W., Falster, D. S., ... & Nakagawa, S. (2020). Sexual dimorphism in trait variability and its eco-evolutionary and statistical implications. elife, 9, e63170.
- Zell, E., Krizan, Z., & Teeter, S. R. (2015). Evaluating gender similarities and differences using metasynthesis. American psychologist, 70(1), 10.
- Zhao, H., DiMarco, M., Ichikawa, K., Boulicault, M., Perret, M., Jillson, K., ... & Richardson, S. S. (2023). Making a ‘sex-difference fact’: Ambien dosing at the interface of policy, regulation, women’s health, and biology. Social Studies of Science, 53(4), 475-494.
- Zugman, A., Alliende, L. M., Medel, V., Bethlehem, R. A., Seidlitz, J., Ringlein, G., ... & Crossley, N. A. (2023). Country-level gender inequality is associated with structural differences in the brains of women and men. Proceedings of the National Academy of Sciences, 120(20), e2218782120.